Standard Analysis of Medical Oxygen Cylinders
As an essential medical device in hospitals, ambulances, and home care settings, the reliability of china oxygen tank valves is directly linked to patient safety and treatment quality. More than just a container, medical oxygen cylinder valves are a life-threatening product. A comprehensive understanding of china oxygen tank standards is essential for manufacturers, global buyers, and medical institutions.
This article will provide an in-depth analysis of four key aspects:
Medical oxygen cylinder dimensions and volumes
Pressure ratings
Valve standards
Related regulations
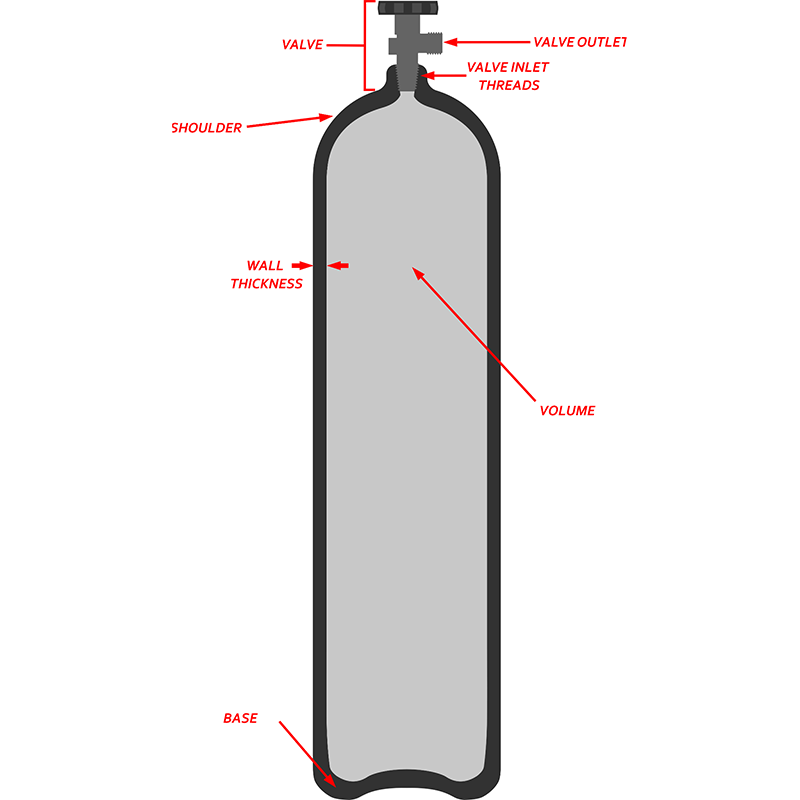
I. Medical Oxygen Cylinder Size and Volume Standards
Medical oxygen cylinder's size and volume directly determine its usage scenarios and convenience.
Common Specifications
Medical oxygen cylinders come in a wide range of capacities, from small, portable 1L–5L bottles, to medium-sized 10L–40L bottles commonly used in hospital wards, and even large 50L cylinders for centralized oxygen supply stations. Different capacities meet different needs:
Small capacity: Suitable for portable use, commonly used for home oxygen therapy and outdoor emergency response;
Medium capacity: Widely used in hospital wards, operating rooms, and rehabilitation centers;
Large capacity: Suitable for ICUs and central oxygen supply systems in large hospitals.
International Standardization Requirements
Common international medical oxygen cylinder standards include ISO 9809 (seamless steel cylinders), EN 1964 (European gas cylinder standard), and DOT-3AA (U.S. Department of Transportation standard). These standards have strict requirements for cylinder diameter, length, weight, and tolerances to ensure global applicability.
Design Considerations
Small medical oxygen cylinders prioritize portability and lightweight design, often using aluminum alloys or carbon fiber composites. Larger medical oxygen cylinders, on the other hand, emphasize high-pressure storage and durability, typically using alloy steel.
II. Pressure Rating Standards
The pressure rating is a key parameter determining the safety of medical oxygen cylinder.
Common Pressure Ranges
The nominal operating pressure of medical oxygen cylinder is typically 15 MPa (150 bar) or 20 MPa (200 bar), with some new high-pressure cylinders reaching 30 MPa (300 bar). High pressure means greater gas storage capacity, but also places higher demands on the cylinder material and manufacturing process.
Testing and Verification
National standards require that china oxygen tank undergo rigorous hydrostatic testing before shipment. The test pressure is typically 1.5 times the operating pressure. This means that a 200 bar china oxygen tank needs to withstand a 300 bar hydrostatic test to ensure leak-free and deformation-free cylinders.
Safety Factor and Lifespan
china oxygen tanks must be designed to meet a certain safety factor (generally ≥2.5). Furthermore, china oxygen tanks require regular inspections, typically every 3–5 years, and must be scrapped if their service life exceeds the limit.
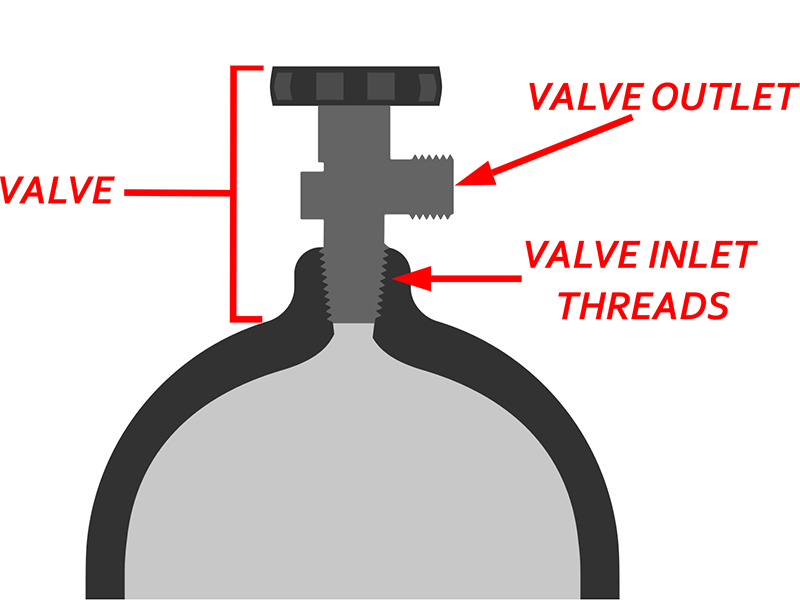
III. Valve Standards and Interface Specifications
Valves are key connecting components between china oxygen tank and respiratory equipment.
Valve Types
Common valve types for china oxygen tank include handwheel valves, pressure reducing valves, and integrated valves. Integrated valves integrate pressure reduction, flow control, and interfaces for greater convenience and safety.
International Interface Standards
ISO 5145 and the Compressed Gas Association (CGA) clearly define valve interface standards for different gases to prevent interoperability.
China oxygen tank typically uses CGA 870 (for small bottles) or CGA 540 (for large bottles) interfaces. DIN 477 is commonly used in Europe.
Safety Design
Medical valves must include anti-misassembly features, backfire prevention, and pressure relief to ensure operator and patient safety.

IV. Relevant Regulations and Compliance Requirements
As a medical device, china oxygen tank is subject to strict regulations in various countries. Snowrain's china oxygen tanks hold multiple certifications and are exported to over 50 countries and regions worldwide, meeting the regulatory requirements of various buyers.
International Regulations
ISO 13485: A quality management system standard for medical devices, requiring manufacturers to have comprehensive quality control processes.
TPED (European Union Transport of Refillable Containers Directive): Specifies requirements for the transportation and refilling of gas cylinders.
FDA (U.S. Food and Drug Administration): Requires approval and registration for medical oxygen cylinders sold in the United States.
Domestic Regulations (China)
The "Regulations on the Safety and Technical Supervision of Gas Cylinders" define the requirements for the entire process of gas cylinder design, manufacturing, filling, use, and disposal.
China oxygen tanks must obtain a medical device registration certificate and be approved by the National Medical Products Administration (NMPA).
Testing and Certification
All medical oxygen cylinders must pass third-party certifications such as CE, ISO, FDA, and SGS before entering the international market. The china oxygen tank produced by snowrain has passed the whole process inspection and obtained multiple international certifications, so buyers can buy with confidence.
Conclusion
China oxygen tank standards cover a wide range of aspects, including dimensions and volume, pressure ratings, valve standards, and regulatory requirements. These specifications not only ensure product safety and reliability during use but also provide a unified basis for global distribution and use. Snowrain's china oxygen tank products undergo rigorous quality control testing before shipment to ensure they meet all factory standards.
As a professional china oxygen tank manufacturer, Snowrain's products strictly adhere to international standards such as ISO, CE, and FDA, and are exported to numerous countries and regions. We welcome global customers to contact us directly at snow@sysnowrain.com for more customized medical oxygen cylinder products. We look forward to collaborating with you.
References:
ISO 9809 – Gas cylinders — Refillable seamless steel gas cylinders
EN 1964 – Transportable gas cylinders — Seamless steel gas cylinders
ISO 5145 – Cylinder valve connections for gas cylinders
State Administration for Market Regulation: "Regulations on Technical Supervision of Gas Cylinder Safety"
FDA – Medical Gas Containers and Closures Guidance
